Promotion of Quality Assurance System
Quality Assurance and Quality Policy

Since that time, as the quality philosophy and practices were deemed to have taken root company-wide, we voluntarily surrendered ISO 9001 certification in December 2013. We have since pursued continuous improvement of quality through the Mandom quality management system.
In 2017, we reviewed our Quality Philosophy and Fundamental Quality Policy, which were revised and reestablished as our Quality Assurance Policy.
Reconfiguring Our Quality Assurance System and Quality Assurance Activities
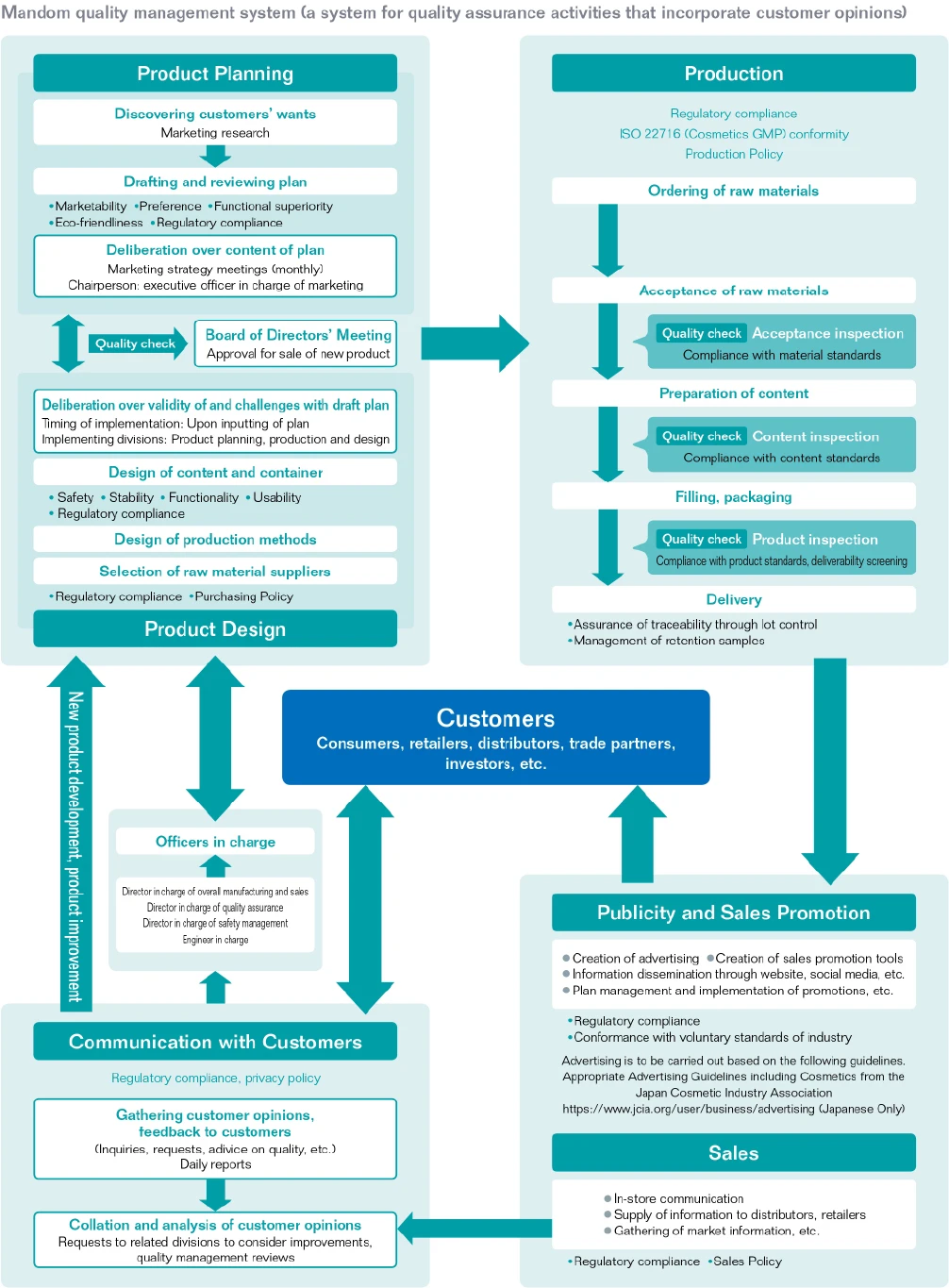
Regarding quality assurance for cosmetics and quasidrugs, Mandom works to assure fully satisfactory quality as demanded by consumers from purchase to end of use. We engage in quality assurance activities to improve the quality of our products and services in all processes, including planning, design and development, production, sales, and customer handling. In January 2014, we established the ISO 9001-based “Mandom quality management system” (a system for quality assurance activities that incorporate customer opinions), which we have since operated as we pursue continuous improvement of quality. In order to continuously improve the effectiveness and reliability of this Mandom quality management system, we established the Quality Assurance Committee through which we unify the company-wide direction on quality. Additionally, the officers in charge, director in charge of overall manufacturing and sales, director in charge of quality assurance, director in charge of safety management and technical supervisors stipulated in the Pharmaceutical and Medical Devices Act (Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices) work closely with each other to supervise and promote quality assurance activities.